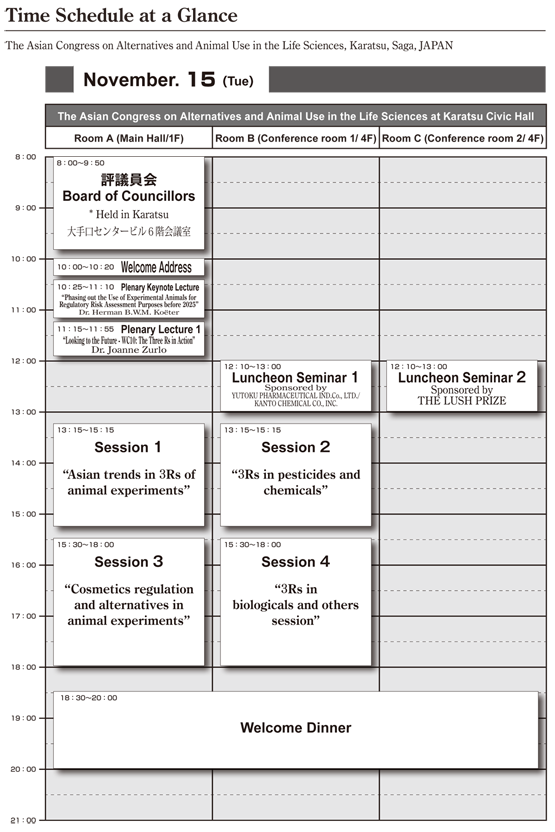
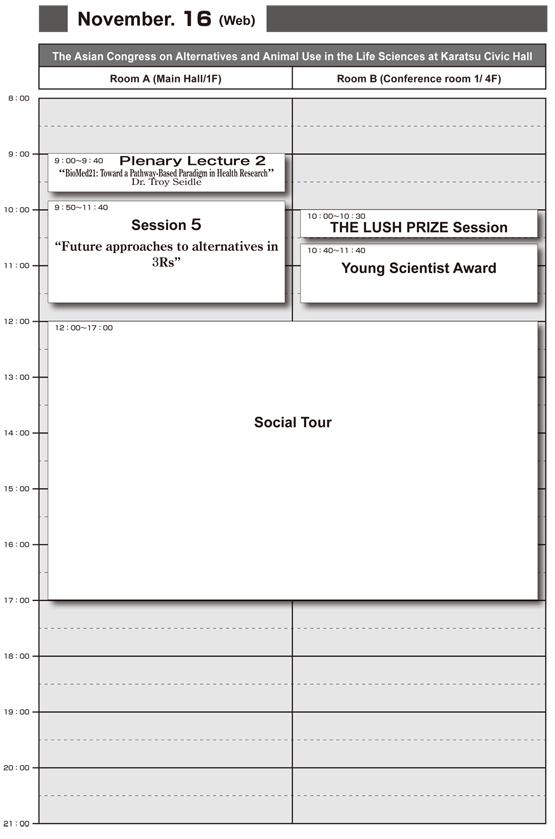
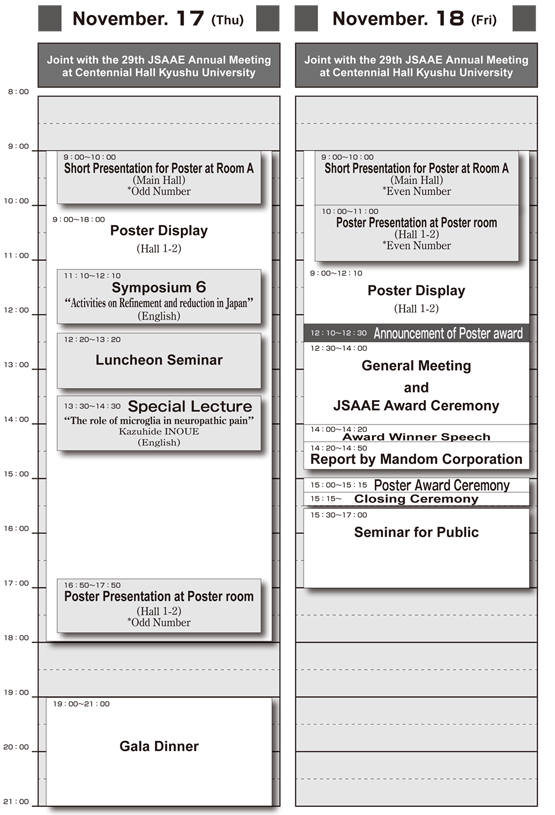
Time Schedule


Program (Correct as of Aug10)
Room A (Main Hall/ 1F) Nov.15, Tue 10:00-10:20
National Institute of Health Sciences (NIHS), Japan
Room A (Main Hall/ 1F) Nov.15, Tue 10:25-11:10
Chair:
Yasuo OHNO
Kihara Memorial Yokohama Foundation for the Advancement of Life Sciences, Japan
Herman B.W.M. KOËTER
Chairman of the Alternatives Congress Trust (ACT), Italy
Room A (Main Hall/ 1F) Nov.15, Tue 11:15-11:55
Chair:
Makoto HAYASHI
makoto international consulting, Japan
Joanne ZURLO
Director of Science Strategy Center for Alternatives to Animal Testing (CAAT),
Johns Hopkins University, USA
Room A (Main Hall/ 1F) Nov.16, Wed 9:00-9:40
Chair:
Noriho TANAKA
Food and Drug Safety Center, Hatano Research Institute, Japan
Troy SEIDLE,et al.
Director of Research & Toxicology Humane Society International, Canada
Room A (Main Hall/ 1F) Nov.15, Tue 13:15-15:15
Chairs:
Tsutomu Miki KUROSAWA
Kagoshima University, Japan
Jufeng WANG
Pharmaron, China
Jae-Hak PARK
Laboratory Animal Medicine,College of Veterinary Medicine,Seoul National University, Republic of Korea
“Trends in 3Rs of laboratory animal experiments (Bio-imaging in Singapore)”
Yi Quan Shawn TAY
Comparative Medicine, National University of Singapore (NUS) / Asian Federation of Laboratory Animal Science Associations (AFLAS), Singapore
“Zebrafish, Danio rerio as a replacement alternative”
Mangala GUNATILAKE
Dept of Physiology,Faculty of Medicine, University of Colombo, Sri Lanka
“The Role of an NGO in Implementing Non-Animal Testing and Training Methods in India”
Dipti M. KAPOOR
PETA India, India
“The Study of In Vitro Skin Sensitization Test Based on Integrating 3D Model to h-CLAT”
Shujun CHENG,et al.
Guangdong Inspection and Quarantine Technology Center, China
“Development of reconstructed human epidermal skin equivalent (EPiTRI) and validation of skin irritation test (SIT) protocol by using EPiTRI”
Huey-min LAI,et al.
Center for Drug Development, Industrial Technology Research Institute, Taiwan
“Preparation and characterization of Reconstructed Human Epidermis (RHE)”
Herlina B. SETIJANTI,et al.
Research Center for Drug and Food, National Agency of Drug and Food Control, Indonesia
“Alternative Research (3Rs) in the World, Asia and Japan”
Tsutomu Miki KUROSAWA
Joint Faculty of Veterinary Kagoshima University, Japan
Room B (Conference room 1/ 4F) Nov.15, Tue 13:15-15:15
Chairs:
Masahiro TAKEYOSHI
Chemicals Assessment and Research Center, Chemicals Evaluation and Research Institute, Japan
Judy STRICKLAND
Integrated Laboratory Systems, Inc. Contractor supporting the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM), USA
Kyung-Min LIM
College of Pharmacology, Ewha Womans University, Republic of Korea
“Approaches to Reducing Animal Use for Acute Toxicity Testing: Retrospective Analyses of Pesticide Data”
Judy STRICKLAND,et al.
Integrated Laboratory Systems, Inc. / Contractor supporting the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM), USA
“3R best practices in pesticide regulation in India”
Alokparna SENGUPTA
Research & Toxicology Department, Humane Society International, India
“Progress in deleting the 1-year dog study for the safety assessment of pesticides”
Horst SPIELMANN
Inst. For Pharmacy, Faculty for Biology, Chemistry and Pharmacy
Freie Universität Berlin, Germany
“Development of Read-across for Chemical Safety Assessment”
Takashi YAMADA,et al.
Division of Risk Assesment, Biological Safety Research Center, National Institute of Health Sciences, Japan
Room A (Main Hall/ 1F) Nov.15, Tue 15:30-18:00
Chairs:
Sanae TAKEUCHI
P&G Innovation Godo Kaisha, Japan
Troy SEIDLE
Humane Society International, Canada
Elian LATI,et al.
BIO-EC LABORATORY, France
“Cosmetic regulation and alternatives to animal experiments in Thailand”
Neti WARANUCH,et al.
Cosmetics and Natural products Research Center, Faculty of Pharmaceutical Sciences, Naresuan University / Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences and Center of Excellence for Innovation in Chemistry, Naresuan University, Thailand
“Cosmetic Regulation and Alternatives in Animal Experiments”
G.N. SINGH
Drugs Controller General (India), Central Drugs Standard Control Organisation, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India
“Cosmetic regulation and alternative in animal experiments in China”
Rong KUANG
Zhejiang Institute for Food and Drug Control, China
“Face Alternative:Current Status in China”
Lu QIU
Shanghai Entry-Exit Inspection and Quarantine Bureau of China, China
“Development of Alternative Test Methods to Evaluate the Safety of Cosmetics”
Tae Sung KIM,et al.
National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Republic of Korea
“Guidance on use of alternative methods for testing in the safety assessment of cosmetics and quasi-drug”
Hajime KOJIMA,et al.
Division of Risk Assesment, Biological Safety Research Center, National Institute of Health Sciences, Japan
Room B (Conference room 1/ 4F) Nov.15, Tue 15:30-18:00
Chairs:
Hiroshi YAMAMOTO
Toyama University, Japan
Katrin SCHUTTE
European Comission DG Environment, Belgium
Chiyoung AHN,et al.
Blood Products Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Republic of Korea
“Alternatives and Refinement for Animal Experimentation in Cancer Research”
Arvind INGLE
Scientific Officer ‘G’, Officer-in-Charge, Laboratory Animal Facility, ACTREC, Tata Memorial Centre, Navi Mumbai, India
Wei YANG
Center for Safety Evaluation, Guangdong Academy of Sciences, China
“Towards global harmonisation of 3Rs in biologicals – efforts of the EPAA Biologicals project team”
Katrin SCHUTTE,et al.
Environment European Comission DG Environment, Belgium
“3Rs in Quality Control of human vaccines: opportunities and barriers”
Sylvie UHLRICH,et al.
Sanofi Pasteur, France
“Veterinary industry approach to 3Rs in Biologicals”
Takeshi FUJII,et al.
Product Development & Regulatory Affairs, Zoetis Japan, Japan
“VICH(International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Products) and Harmonization of Criteria to Waive TABST(Target Animal Batch Safety Test) for Vaccines for Veterinary Use”
Koji OISHI
National Veterinary Assay Laboratory, JMAFF, Japan
Room A (Main Hall/ 1F) Nov.16, Wed 9:50-11:40
Chairs:
Yasuyuki SAKAI
Tokyo University, Japan
Mohammad A. AKBARSHA
Bharathidasan University, India
Catherine WILLETT
Humane Society of the United States / Humane Society International, USA
“Progress of alternative study in China”
Jufeng WANG
Pharmaron, China
“Mechanism based evaluation system for hepato- and nephrotoxicity or carcinogenicity using omics technology”
Fumiyo SAITO
Chemicals Assessment and Research Center, Chemicals Evaluation and Research Institute, Japan
“Stem cells and alternatives”
Eui-Bae JEUNG
College of Veterinary Medicine, Chungbuk National University, Republic of Korea
“Futuristic approach to alternative model organisms: Hydra stakes its claim”
Mohammad A. AKBARSHA,et al.
Mahatma Gandhi - Doerenkamp Center for Alternatives to Use of Animals in Life Science Education, Bharathidasan University, India
(Sponsored by Japan Chemical Industry Association, Japan Anti-Vivisection Association, Japanese Society for Alternatives to Animal Experiments)
Room B (Conference room 1/ 4F) Nov.16, Wed 10:40-11:40
Chairs:
Hiroaki TODO
Faculty of Pharmaceutical Sciences, Josai University, Japan
Horst SPIELMANN
Inst. For Pharmacy, Faculty for Biology, Chemistry and Pharmacy Freie Universität Berlin, Germany
“Evaluation of toxicants the neural differentiation of human ESCs”
Jin Yong AN,et al.
Chungbuk National University, Republic of Korea
JAVA Award
“Preliminary Evaluation of Vascular Endothelial Growth Factor as a Biomarker for Alternative Skin Sensitization Test in HaCaT Keratinocytes”
Sae On KIM,et al.
College of Pharmacy, Seoul National University, Republic of Korea
JSAAE Award
“The effect on cellular function and microstructures of hepatic spheroids by using a novel method for loading ECM thin layer into spheroids”
Fumiya TAO,et al.
Yokohama City University, Japan
(Sponsored by THE LUSH PRIZE)
Room B (Conference room 1/ 4F) Nov.16, Wed 10:00-10:30
Carol TREASURE, et al.
XCellR8 Ltd. UK
(Sponsored by YUTOKU PHARMACEUTICAL IND.Co., LTD.
KANTO CHEMICAL CO., INC.)
Room B (Conference room 1/ 4F) Nov.15, Tue 12:10-13:00
Shigehisa AOKI
Department of Pathology & Microbiology, Saga University Japan
(Sponsored by THE LUSH PRIZE)
Room C (Conference room 2/ 4F) Nov.15, Tue 12:10-13:00
Rebecca RAM
Scientific Consultant, Lush Prize, UK
(Sponsored by Mandom Corporation)
Room B (Conference room 1/ 4F) Nov.14, Mon 14:00-17:00
Humane Society of the United States / Humane Society International, USA
Kellie FAY
US Environmental Protection Agency, USA